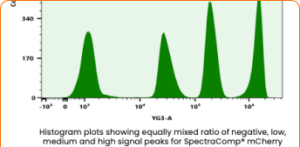
Unlocking the Power of TruCytes™ for Next-Generation Therapeutics
As the cell therapy landscape evolves, regulatory oversight is becoming more stringent. FDA regulation 21 CFR Part 1271 urges cell therapy manufacturers to address challenges like sterility, reproducibility of product lots, and control of impurities during the production process. The FDA also mandates potency and viability testing, ensuring that each product batch meets robust quality standards. […]