The race to bring new drugs and cell therapies like CAR-T to market is more intense than ever. Patients are waiting, and developers face mounting pressure to accelerate timelines, reduce costs, and meet evolving regulatory demands—all without compromising quality.
Unfortunately, commonly used controls like engineered cell lines or donor-derived blood cells introduce variability and operational challenges, which compound an already complex ecosystem instead of helping to manage it. The lack of consistent assay benchmarks can create roadblocks across the entire development pipeline—from discovery to clinical trials, to commercial production after market approval.
So, how can we work smarter and faster without compromising quality?
The answer lies in precision-engineered cell mimics.
What Are Cell Mimics?
Cell mimics are designed to accurately replicate specific characteristics and functions of biological cells, without being alive. They can be readily customized and adapted for therapeutic and diagnostic applications.
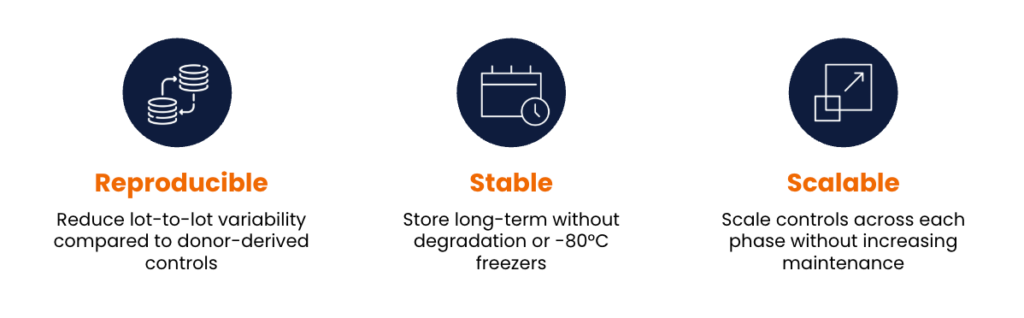
Key features like size, granularity, and surface molecules—including biomarkers and fluorophores—can be precisely tuned to mimic different cell types. This tunability gives cell mimics a major edge over biological cells, providing significant time and cost advantages in the development, characterization, and clinical evaluation of cell therapies.
How Cell Mimic Controls Improve CAR-T Assay Efficiency
Flow cytometry is a critical tool in CAR-T cell therapy development, used to assess phenotypic markers, quantify T cell transduction and measure CAR expression. To ensure assay accuracy, many labs rely on biological controls like donor-derived PBMCs or in-house transduced CAR-T cells.
However, developing and maintaining in-house controls comes at a cost. Sourcing donor materials, investing in cell line development and maintenance for analytical quality control (QC) standards requires substantial time, labor and technical expertise—resources that are diverted away from R&D efforts that advance therapeutic programs.
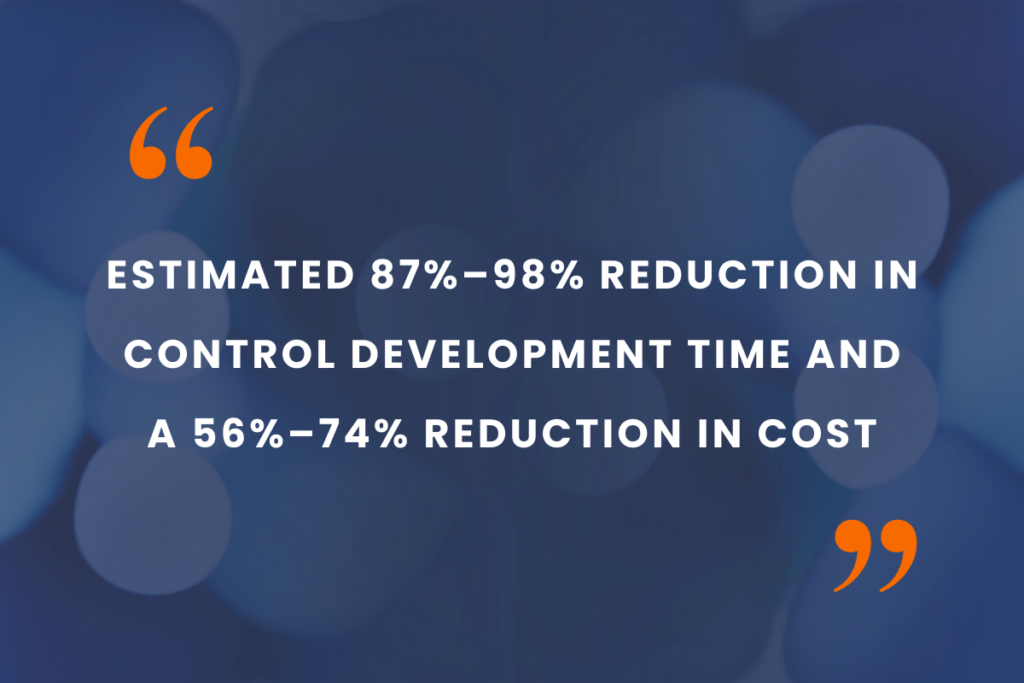
Slingshot Biosciences’ scalable and reliable cell mimic controls provide an estimated 87%–98% reduction in control development time and a 56%–74% reduction in cost compared to traditional biological controls like donor-derived materials or in-house cell lines. These savings reflect both the elimination of time-intensive culturing and qualification steps, as well as reduced ongoing maintenance and sourcing costs throughout the cell therapy workflow.
Cell Mimics in Action
For the Fred Hutch Cellular Processing Facility QC lab (CPF QC), custom CD3 T cell mimics engineered to maintain stable expression of the common CAR transduction marker epidermal growth factor receptor (EGFR) were implemented to validate CAR-T cell flow cytometry assays. Unlike their in-house transduced CAR T cells, the cell mimics provided a more stable and reproducible reference material that could be used throughout development and clinical testing.
By integrating cell mimics into their CAR-T enumeration assays, Fred Hutch scientists estimate that development costs could be reduced by 60–75% and valuable FTE hours previously spent on cell maintenance could be reallocated to higher-value activities.
Advancing the Future of CAR-T Therapies
Cell mimics offer a smarter, faster, and more cost-effective alternative to biological controls. With consistent, stable, and reproducible reference materials, scientists can overcome the limitations of biological cells, improve quality control standards, and streamline development timelines.
Ultimately, these time- and cost-saving efficiencies will help developers bring life-saving cell therapies to patients faster, advancing the field of CAR-T development and transforming the future of medicine.
Considering how to leverage cell mimics in your CAR-T program?
Request an initial call with our team to explore what’s possible