Get reliable, reproducible results in every cell therapy phase with custom biomarker cell mimic controls.

Eliminate the inconsistencies of traditional controls with a custom biomarker cell mimic that delivers unmatched precision for cell therapy testing:
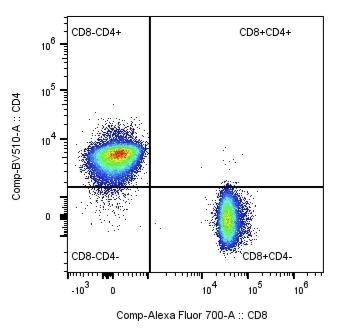
Monitor low-frequency CAR-T cells with confidence. Our custom biomarkers provide the critical data to measure treatment efficacy:
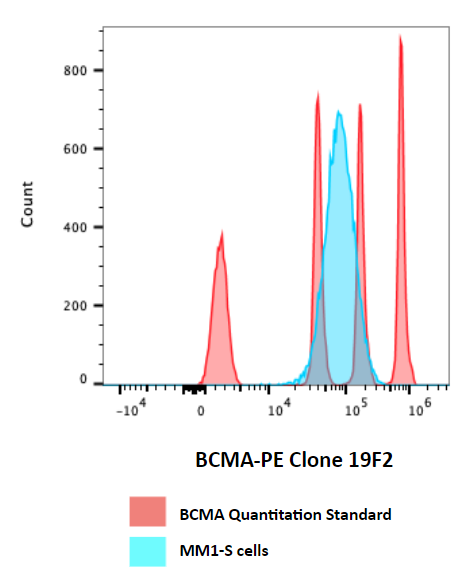
Our custom biomarker controls consistently outperform both biological and plastic bead controls across fluorescence, light scattering, and biomarker compatibility. Replace maintenance and variability with a predictable, ready-to-use solution.
Biological Controls

Polystyrene Beads


Slingshot Bio teamed up with Cerba Research to develop cell mimics for Ki-67, a protein vital to cancer research. The solution allowed Cerba to validate assays with greater accuracy and less variability than what was possible with live cells, improving consistency in results.
“Slingshot Biosciences can generate controls that truly mimic biological expression. It’s a game changer for our project … These cell mimics have the potential to fill gaps in rare marker controls needed for assay validation.”
Dr. Veronica Nash | US Regional Head of Flow Cytometry
Cerba Research

See how we helped Prolocor design the ideal control for their platelet FcγRIIa precision diagnostic test that quantifies FcγRIIa on the surface of platelets to guide clinical decision making.
“Clinicians need better tools to guide decision making on the choice of antiplatelet therapy in coronary artery disease patients, particularly after coronary stenting. The Prolocor pFCG® test will be an important asset as we tailor antiplatelet therapies to balance thrombotic and bleeding risk.”
Dominick J. Angiolillo, MD, PhD, FACC, FESC, FSCAI | Professor of Medicine, Chief, Division of Cardiology, Director, Thrombosis Research Center
University of Florida, College of Medicine-Jacksonville.

See how we partnered with Fred Hutch CPF QC to engineer custom CD3 T cell mimics with varying percentages of expression of the common CAR transduction marker epidermal growth factor receptor (EGFR) to validate their CAR T cell flow cytometry assays.
“In our search for reliable controls for cell therapy assays, partnering with Slingshot has been a game-changer. Together, we addressed significant challenges by engineering custom CD3 T-cell mimics.”
Amina Kariminia, Ph.D. | Immunologist, Analytical Development Scientist
Fred Hutch Cancer Center

TruCytes™ Biomarker Controls
Replicate and quantify cell types with customizable proteins for cell therapy applications.

ViaComp Cell Health Controls
Ensure accurate cell viability measurements with controls for DNA-intercalating and amine-reactive dyes.

SpectraComp Compensation Controls
Get accurate compensation and unmixing for assays with controls that stain like real cells.

FlowCyte Calibration Controls
Set new standards for instrument calibration, ensuring consistent results across labs.
Our polymer-based biomarker cell mimic controls can replicate specific cell characteristics for a range of controls needs, including patient monitoring, product characterization, and therapeutic validation.
By using this website, you agree to the storing of cookies on your device. These cookies are used to collect information about how you interact with our website and allow us to remember you. We use this information in order to improve and customize your browsing experience and for analytics and metrics about our visitors both on this website and other media. To find out more about the cookies we use, see our Cookie Policy.
synthetic cell controls, slingshot biosciences incorporated, slingshot biosciences, trucytes synthetic cell controls, slingshot bioscience
Customizable biomarker controls offer significant advantages in cell therapy research, including enhanced precision and tailored solutions for specific experimental needs. These controls allow researchers to design experiments that align closely with their unique therapeutic goals, ensuring more reliable and reproducible results.
For instance, by utilizing custom biomarker controls, researchers can achieve specific antigen densities and mimic various cell types, which is crucial for accurately characterizing cell therapies. This approach not only improves the quality of data obtained but also accelerates the development of personalized treatment strategies.
Real-world applications of Slingshot's customizable biomarker controls demonstrate their effectiveness in overcoming challenges faced in cell therapy research. Case studies from leading institutions showcase how these products have transformed experimental outcomes, providing compelling evidence of their utility.
For example, researchers at the Fred Hutch Cancer Center reported significant improvements in assay reliability after integrating Slingshot's CD3 T-cell mimics into their workflow. These success stories illustrate the practical benefits of using advanced biomarker controls in clinical and research settings.
The technology behind biomarker cell mimics is rooted in advanced polymer science, enabling the replication of complex cell characteristics. This scientific foundation allows for the creation of highly accurate models that can be tailored to specific research needs, enhancing the overall quality of cell therapy studies.
By utilizing polymer-based systems, Slingshot's biomarker mimics can effectively simulate the behavior of actual cells in various environments, providing researchers with valuable insights into cellular responses and interactions. This depth of understanding is essential for developing effective therapies and improving patient outcomes.
Slingshot Biosciences encourages collaboration with researchers to develop customized solutions that meet specific experimental requirements. Engaging with experts in the field can lead to innovative approaches and optimized results in cell therapy research.
Through consultations and personalized support, Slingshot offers guidance on selecting the right biomarker controls for various applications, ensuring that researchers can achieve their desired outcomes efficiently. This partnership approach fosters a collaborative environment, enhancing the overall research experience.