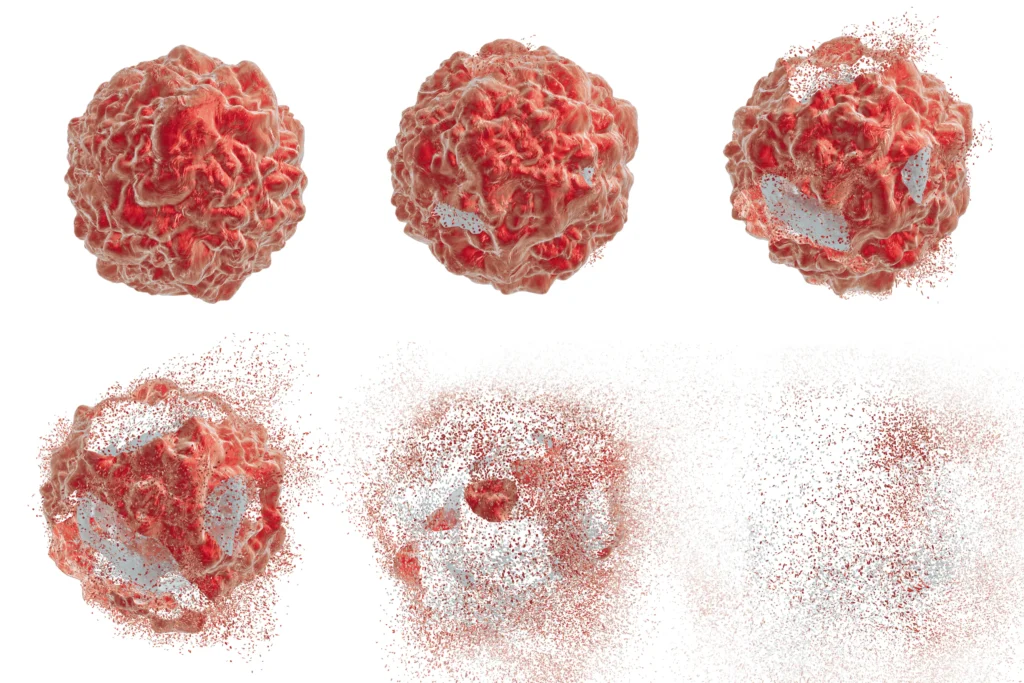
Cell health is a critical parameter at multiple stages of the cell therapy process, ensuring that the cellular product is safe, functional, and of the highest therapeutic quality.
The health of cells used in therapy is not just a metric; it’s a cornerstone of the therapy’s potential success. Ensuring that cells are healthy, functional, and safe benefits both the patient and the broader medical community. In addition to supporting therapeutic efficacy and patient safety, monitoring cell health throughout the cell therapy process also impacts meeting regulatory and quality standards and can have implications for optimizing manufacturing and improving cost efficiency.
Whether cells are sourced from a patient (autologous) or a donor (allogeneic), the initial cell collection’s quality can significantly influence the end product. Viability staining and basic phenotyping for proliferation markers such as Ki-67 can determine the health and composition of the initial cell sample. After collection, cells often undergo expansion in culture to generate the necessary quantities for therapeutic doses. Periodic checks for viability, growth rate and metabolic health can be helpful as prolonged culture can induce stress, leading to decreased cell viability and altered functionality.
Post modification cells are checked for viability and functional assays may be conducted to confirm successful genetic integration without compromising cell health. After expansion and any necessary modifications, cells are harvested and formulated for patient administration. However, the harvesting process involves enzymatic or mechanical detachment and the subsequent washing steps, can be stressful and impact cell health. Viability and apoptosis assays are vital at this stage. Additionally, checks for cellular aggregation or debris can indicate potential issues.
“The ViaComp separates allows consistency at distinguishing positive and negative populations with dimmer viability dyes on full-spectrum instruments and has produced better staining efficiency than the competitor product.”
Robert Ruidera, Scientist II – Immunology, Marengo Therapeutics
Many cell therapies are cryopreserved for storage and transportation and are later thawed before administration to patients. Cryopreservation can introduce cellular stress, and the thawing process can further affect cell health. Post-thaw viability assessments and functional assays ensure the cells remain in optimal health and retain therapeutic efficacy.
Finally at product release, ensuring the product meets all regulatory and safety standards requires a combination of viability, phenotypic characterization and functional assays.
Almost every step of the cell therapy process requires careful consideration of cell health. Such vigilance ensures that the therapeutic product is of the highest quality, thereby maximizing patient outcomes.
One often overlooked step in assessing cell health is the need for relevant cellular controls, which can be difficult to generate in-house but are necessary for developing a quality therapeutic product. Slingshot leverages the tenets of biochemistry, high-precision manufacturing and polymer chemistry to engineer cell mimics that can replace biological controls as reference standards.
Slingshot Biosciences Cell Health Portfolio contains necessary controls for viability assessment, toxicity studies as well as proliferation assessments to support cell therapy developers in creating effective therapeutics.